In
ancient medicine, the herb, Cannabis sativa L., was widely used to
cure disturbances and inflammation of the bowel. Research published
in the journal Pharmacology and by the United States (US) National
Institute of Health has found that cannabis is effective in treating
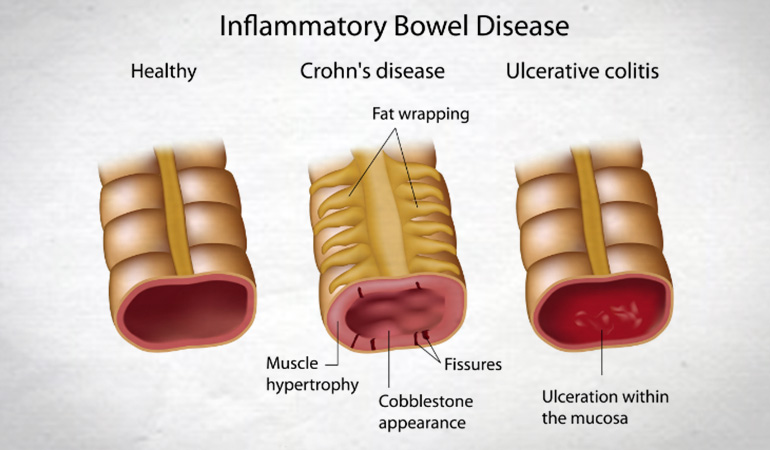
Crohn’s Disease, which is a form of inflammatory bowel disease
(IBD). IBD's such as Ulcerative Colitis and Crohn’s Disease affect
over a million people in the US. Many IBD victims suffer
from extreme pain, diarrhoea and poor ability to digest food. Up to
half of IBD cases are so severe that they ultimately require surgery
to remove the affected bowel segment.
In a 2007 study in Australia, The Economic Costs of Crohn’s Disease and Ulcerative Colitis, commissioned by Crohn’s and Colitis Australia (CCA) it was revealed that the (then) annual cost of Crohn’s Disease and Ulcerative Colitis was AU$2.7 billion. In 2013, CCA commissioned another report, Improving Inflammatory Bowel Disease Care Across Australia, which stated: "IBD is becoming more prevalent, more complex, and more severe ... IBD is a chronic and largely hidden disease affecting approximately 1 in 250 people aged 5 – 49 nationally. Australia has one of the highest rates of prevalence and incidence in the world and each year more and more young people are being diagnosed. Over 74,955 Australians are burdened with a constant and often hidden struggle that affects a sufferer’s personal, social and work life". The report went on to estimate that national total hospital costs for IBD are in the order of AU$100 million per annum. Productivity losses attributable to IBD in 2012 were estimated at over AU$380 million. An additional AU$2.7 billion of financial and economic costs have been associated with the management of IBD, Australia-wide.
In a 2007 study in Australia, The Economic Costs of Crohn’s Disease and Ulcerative Colitis, commissioned by Crohn’s and Colitis Australia (CCA) it was revealed that the (then) annual cost of Crohn’s Disease and Ulcerative Colitis was AU$2.7 billion. In 2013, CCA commissioned another report, Improving Inflammatory Bowel Disease Care Across Australia, which stated: "IBD is becoming more prevalent, more complex, and more severe ... IBD is a chronic and largely hidden disease affecting approximately 1 in 250 people aged 5 – 49 nationally. Australia has one of the highest rates of prevalence and incidence in the world and each year more and more young people are being diagnosed. Over 74,955 Australians are burdened with a constant and often hidden struggle that affects a sufferer’s personal, social and work life". The report went on to estimate that national total hospital costs for IBD are in the order of AU$100 million per annum. Productivity losses attributable to IBD in 2012 were estimated at over AU$380 million. An additional AU$2.7 billion of financial and economic costs have been associated with the management of IBD, Australia-wide.
The past decade has seen a constant rise in publications dealing with the anti-inflammatory effects of cannabinoids and the potential underlying mechanisms. Preclinical data on the ameliorating effect of synthetic and natural cannabinoids in animal models mimicking features of IBD have been rapidly evolving. The reasonable idea that cannabinoids would also be beneficial in IBD patients was mainly based on results from experiments in cannabinoid receptor knock-out mice and on data using cannabinoid receptor agonists and antagonists.
In 2011, a retrospective, observational study examining disease activity, use of medication, need for surgery and hospitalisation before and after cannabis use in 30 patients (26 males) with Crohn's Disease and a questionnaire performed by a different group of patients with Ulcerative Colitis (100) and Crohn's Disease (191), both revealed symptom relief and improvement after use of cannabis. 21 out of 30 of the study individuals reported significant improvement, with patients requiring steroid treatment reduced from 26 to 4.
 A prospective trial in Israel showed complete remission in five of eleven patients suffering Crohn's Disease who were given cannabis twice daily. Authors of the study said it had been reported for years that cannabis lessened the painful symptoms of the inflammatory bowel disease, but the findings had not been proven in a controlled trial. The study, published in Clinical Gastroenterology and Hepatology in 2013, compared 21 patients who did not respond to conventional treatment. Half were given cannabis cigarettes and the other half were given a placebo; cannabis cigarettes with the tetrahydrocannabinol (THC) removed. The results showed improvement in the group treated with the THC-intact cannabis. Those subjects also reported improved sleep and appetite.
A prospective trial in Israel showed complete remission in five of eleven patients suffering Crohn's Disease who were given cannabis twice daily. Authors of the study said it had been reported for years that cannabis lessened the painful symptoms of the inflammatory bowel disease, but the findings had not been proven in a controlled trial. The study, published in Clinical Gastroenterology and Hepatology in 2013, compared 21 patients who did not respond to conventional treatment. Half were given cannabis cigarettes and the other half were given a placebo; cannabis cigarettes with the tetrahydrocannabinol (THC) removed. The results showed improvement in the group treated with the THC-intact cannabis. Those subjects also reported improved sleep and appetite.The 8-week treatment with THC-rich cannabis caused a decrease in the Crohn's Disease activity index in 90% of patients without producing significant side effects. The mechanisms involved most likely include peripheral actions on cannabinoid receptors 1 and 2 (CB1 and CB2) and may also include central actions. The authors rightfully concluded that a larger patient group is warranted for future studies.
The wall of the gastrointestinal tract houses all components of the ECS. Data from 2011 showed that these components are differentially expressed in human IBD indicating a regulatory role in the disease progression. While anandamide and its synthesising enzyme display lower levels in Ulcerative Colitis, expression of CB2 receptors and enzymes responsible for synthesis and degradation of 2-AG were increased (from data in 2009). The findings indicate that the CB2 receptor plays a key role in the ameliorating effect of cannabinoids in IBD. The precise mechanism as to how cannabinoids contribute to the improvement of IBD, however, is not clear but by use of experimental models of intestinal inflammation we are able to define a picture on how and at which targets cannabinoids cause improvement of inflammation.
The primary mechanisms through which cannabis exhibits healing properties in Crohn's Disease are its immuno-modulatory and anti-inflammatory properties:






No comments:
Post a Comment